-
Title (Dublin Core)
-
Chemists on the front
-
Article Title and/or Image Caption (Dublin Core)
-
Title: Chemists fight on 1000 fronts
-
extracted text (Extract Text)
-
LIKE you and me and 130,000,000 others,
the American chemist is in this war
to win. But unlike most of us, he's fight-
ing it on a thousand fronts at once. He
sees that Marines pushing forward in the
South Pacific have more punch in their
ammunition, that our planes have the extra
snap and drive of high-octane gasoline,
that there is TNT in those 8,000-pound
bombs that drop on German war plants. At
the same time it’s his job to see that Tom
Jones, defense worker, doesn’t get sick
from exposure because he can't get a wool
overcoat, a rubberized raincoat, or medi-
cines. And that Mrs. Jones’ table has va-
riety even though tin is needed for war
industry and can’t be spared for cans.
This war is being fought by billions of
people using hundreds of thousands of
things. It's being fought with everything
from high explosives and heavy metals to
that tiny bit of plastic that goes into the
tips of your shoelaces in order to save
that much more metal for ships and tanks.
Thousands of those things are born in
the test tube or under the microscope. The
chemist has been drafted for the duration.
His job is as vast as global war: new, bet-
ter, quicker ways of making the old things
... new things to take the place of the old
where shortages threaten war production
or civilian comfort.
For chemistry at war doesn’t mean just
poison gases like chlorine, phosgene, or
lewisite. It doesn't mean ‘secret weapons.”
It means new landing fields for fighting
planes created overnight. Chemistry does
that. Barrels of dilute sodium silicate
poured over raw meadowland toughens the
ground and saves weeks of digging, of haul-
ing crushed stone for a foundation. Then
a layer of new, quick-setting, tougher as-
phalt, and your war birds can hit the tar-
mac at 150 miles an hour with no fear
of a crack-up.
It means tanks, trucks, and jeeps rolling
forward under cover of a smoke screen
made with chlorsulphonic acid. And the
chemist had to find something to take the
place of the chlorine that whitened your
linen or bleached the pulp for your morning
newspaper, to scrape together all the chlor-
ine needed at the front.
The foot soldier behind those tanks owes
a big debt to the chemist. The lining of his
combat helmet is not metal. The stock of
his Tommy gun is not wood. Both are
phenol formaldehyde plastics compounded
out of the same chemicals.
He may smash through enemy lines. The
advance may carry him two or three days’
march ahead of his field kitchens. But in
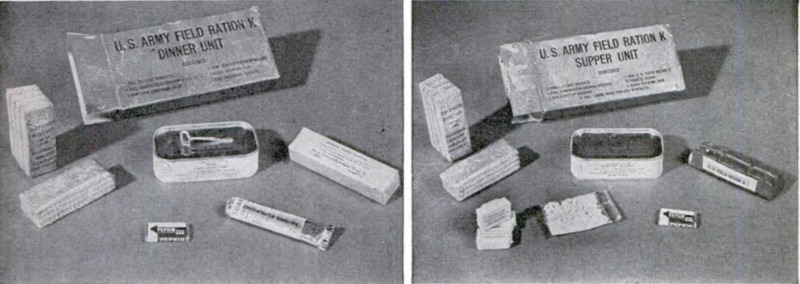
his pack your doughboy carries several ob-
long packets of Ration K. Each contains
the equivalent of three full meals—a day's
rations, in a container the size of a sample
box of breakfast food!
Special goggles protect his eyes against
the glare of desert sands or arctic snows.
And if the lenses -should become scratched
or dented, he merely throws them away and
slips in another pair. Molded out of a light-
polarizing, nonshatterable vinyl polymer,
they are produced in a fraction of the time
and at a fraction of the cost of grinding
glass lenses. Best of all, eyeglasses are no
longer a danger to the soldier in battle.
Chemistry found the answer to that prob- |
lem and to many others—Ilike the danger
of death from fire in a plane set ablaze by
enemy incendiary bullets. Today your
fighter pilot's flying suit may char, but it
will not burn. It has been made flameproof
with tricesyl phosphate or with ammonium
sulphamate, |
In field and base hospital, chemistry plays
a major part. Army medical men are be-
ginning now to talk about our “victory” at
Pearl Harbor—the victory of sulfanilamide
and sulfathiazole, made in the U.S.A. But
the chemist has already passed beyond the
miracle of the sulfa drugs. Synthetic urea,
long used in making plastics, fertilizers, and
beverage alcohol, is expected to save thou-
sands of lives through a new technique for
the treatment of wounds. |
Malaria is an enemy more deadly than the
combined Axis armies. Each year it kills
more victims than died on both sides in the |
last World War. Without quinine, or some
other weapon against malaria, our soldiers
could not hold out for a month in the South
Pacific, Africa, or India. Our major sources
of quinine are in Japanese hands today.
Yet, less than a year after Pearl Harbor,
three new American-made drugs, plasmo-
quin, atebrin, and totaquine, are competing
for the honor of filling quinine’s shoes. The
first two are synthetic quinines. The third
is made, like quinine, from cinchona bark. |
South America cannot supply us with
enough cinchona bark to give us the quinine
we need. But it can ship us enough to make
a little quinine, plus plenty of totaquine. |
That's good news not only for our fighting
men in tropical countries, but for something
like 1,000,000 civilians in the United States
who have malaria today.
These jobs sound important enough, and
thousands of them add up to big figures on
the score card of total war. But there are
far bigger jobs.
Let’s look at the new heavy artillery of
American chemistry at war. These big guns
are the latest fractional distillation towers
and cracking plants for breaking down
crude oil into many vital war materials.
More impressive than the biggest blast fur-
naces of the steel age, the huge batteries of
sleek, cylindrical columns are like cannon
aimed to shoot half way round the world—
which is just what they are doing.
‘Within two years, they will be turning
out enough butadiene to give us more than
1,000,000 tons of synthetic rubber. From
those towers, instead of the plantations of
Malaya, will come the tires for our trucks,
tanks, planes, pontons, self-sealing gaso-
line tanks, and gas masks—as well as for
our own aging cars.
From those same heavy batteries will pour
motor fuel—100-octane and far higher if
our engineers can design motors to make
full use of such gasoline—light oil; benzene;
acetylene for plastics, rubber, and synthetic
textiles; and toluene.
Suppose a chemist had walked into the
G.H.Q. of the Allied High Command in 1917
and said: “Gentlemen, give me
plenty of petroleum—just plain crude
oil—and money to build plants, and
I'll guarantee you as much TNT as
you can use!” What would have hap-
pened? The august generals would
have shipped him off to an asylum.
Trinitrotoluene — innocent-looking
pale, yellow crystals made up of car-
bon, hydrogen, oxygen, and nitrogen
—is the demon destroyer of modern
war. TNT is made from toluene and
nitric acid. Step it up with more
nitrogen in the form of ammonium
nitrate and you have amatol, another
deadly explosive.
In World War I the Allies had to
depend for nitrogen compounds on
natural nitrates, chiefly Chile salt-
peter. These had to be mined, lugged
hundreds of miles to the seacoast, loaded
onto ships, Those ships—thousands of them
—threaded their way painfully across the
world's oceans at six or eight knots. Then,
unless they were sent to the bottom by Ger-
man subs the nitrates were unloaded,
shipped again, refined, treated with sul:
phurlc acid, and finally made into nitric
acid for explosives.
Toluene, the other partner in the combi
‘nation, has a romantic history. Discovered
by a French chemist just 100 years ago in
the balsam of the folu, tree that grow in
South America, it was later obtained from
the balsam of an static palm, called "Drag-
on's Blood.” Ita chief use then was to give
a pleasant taste to cough sirups. But before
it found its way into the manufacture of
high explosives, toluene was being recovered
trom coal tar.
At that particular job, the Allies were
hopelessly outclassed. The chemists of Ger-
many were years ahead in breaking down
that black, sticky mass called coal tar, a
by-product of the coke ovens that furnished
fuel to her enormous steel Industry. From
i, the Germans had bullt up a long list of
synthetic dyes and drugs, and they were
ready to turn their skill to more deadly
work.
‘But the American dye industry was in its
infancy 25 years ago. It could turn out no
more than a fraction of the toluene needed
by the Allies.
"Yet since Pearl Harbor the rash promise
of “all the TNT you can use” has been made
by American chemists. And it wil be kept.
For American petroleum chemists have
learned to make toluene from crude ofl far
faster than it can bo synthesized from coal
tar. When our plants are finished, toluene
‘will pour out in quantities to match our new
‘production of nitrogen, which wil literally
be snatched out of the alr through the high-
pressure synthesis of ammonia!
High-octane gasoline ia really the grand-
father of the TNT bombs that drop on Ger-
many today—a grandfather only five years
old! It was while seeking means of making
more high-octane fuel that petroleum
chemists discovered they could break crude
oil down and reshape it into a host of other
things. In working for more and better
high-octane, they learned to make hydro-
carbon, ethyl and methyl alcohol, and other
carbon‘ hydrogen-oxygen compounds that
are tho bricks and stones from which are
bullt lacquers, paints, varnishes, and sol-
vents; rayons and plastics; dyestuffs, tex-
tile and leather olla; synthetic rubber; medi-
cines, chloroform, ‘poisons, toiletries, tear
gas, poison gas, vitamins, soupless soaps, a.
spray to ripen frreen frult after it has been
plcked—and toluene! They discovered that
it 1s possible to make from crude oil many
things that can be obtained from coal tar.
Not only in big things are our battles
being fought and won in American labora-
tories. Some of the lesser skirmishes are
nearly as Important and just as Interesting.
arly in the defense program the Army's
ordnance men realized that a shortage of
brass would hold up the manufacture of
cartridge cases. Unlimited TNT would win
Bo wars unless we had the cartridges. To
make the cases out of thin-gauge steel was
easy. Copper plating would take care of
the outside. But the inside must withstand
nitrocellulose, nitroglycerin, acetic acid, am-
‘monium hydroxide, ethyl alcohol, and ethyl
other. If it in coated, the coating must re-
sist extremes of heat and cold. It is worth-
leas if it scratches or chips off when the
shell is loaded. The chemists of Frankford
Arsenal found the answer In a straight,
water-clear, phenol formaldehyde baked-on
finish. Yes, you skeet shooters and rifle
fans, the day of dependence on expensive
brass for cartridges is past.
Armies that have to fight in all kinds of
climates use staggering quantities of wool.
It would take nearly the entire wool clip of
the United States for two years to outfit
every soldier in our Army, not counting
civilians or the troops of our allies! Yet
the spoilage of woolen goods is tremendous,
as every housewife knows. One female
moth and her descendants can, in a single
year, destroy as much wool as 13 sheep can
produce! Soap alkalis ruin wool, while cer-
tain enzymes decompose it and some bac-
teria seem to thrive on it.
IN ITS molecular structure, wool differs
from all other fibers. Long chains of mole-
cules are joined together by other, weaker
chains, sprouting in all three dimensions.
It Is these weak cross links which are bro-
ken down by the moths digestive juices or
the soap alkalis, so that the tougher chain
Sbers fal apart,
Chemists of the Bureau of Standards
have an answer to the problem. They do
the moths work for her. They dissolve the
weak disulphide or cystine bonds of the
wool with soluble organic sulphur com-
pounds known as mercaptans. Then they
replace the severed bonds with a chemically
stable material through the use of a re-
agent like methyl or ethyl dibromide. The
chemical reagent builds new cross links that
are proof against moths, alkalis, or bacteria.
And 50 you have wool that is mothproof and
can safely be washed with ordinary soap!
Jot this down in your notebook for V-day.
The woolen suit you buy after the war may
De a trifle more expensive, but it will outlast
the old one four to one. And no expensive
dry cleaning. Tl go right to the laundry
with your socks.
I¢ you stand on the sidelines, it looks as
though American chemists sprang suddenly
to life on December 7, and have been fran-
tically pulling rabbits out of hats with both
hands ever since. It is true that they have
crammed into a few months progress that
would normally have taken years. But look-
ing at that, and that alone, does not give a
fair picture.
When guns stopped talking in 1919, our
chemists began thelr conquests whers our
Soldiers had left theirs. They took from
Germany her leadership in the synthetic-
dye industry. They gave us improvements
in stainless steel and & host of other im-
provements in heavy and light metals. Re-
search on silicates let the sunlight into our
skyscrapers through walls of glass brick;
developments in refrigerants and insulators.
gave us alr conditioning and quick-freeze
food storage, and freed us from lifelong
servitude to the whims of climate.
We got a whole world of new plastics
out of coal, air, and water; synthetic rub-
ber; aluminum ‘in our planes, automobiles,
railway cars and kitchen stoves; practically
indestructible finish on our cars; high-oc-
tane gasoline, neon lighting, nylon stock-
ings, rayon underwear, vitamins that
streamlined our eating, and sulfa drugs
that revolutionized medicine.
Our chemists were producing for peace,
not war. But they were training men, per-
fecting techniques, building a vast machine
that needed only a signal to slip into high
gear for war production if necessary. The
petroleum industry alone had built up an
army of 6,00 research workers.
That's why, by the end of next year, we'll
be producing seven times as much alumi-
num as we made in 1939—enough to build,
in one year, three times the number of
passenger cars on all American railroads.
That's why, with new plants designed to
produce magnesium from sea water, we can
afford to put half a ton of that light metal
into every fighting plane we build. That's
why attack gliders and training planes of
wood are rolling off the production lines
right now. With years of experience with
synthetic resins, chemists were able to pro-
duce phenol formaldehyde binders and fin-
ishee to cement layers of wood veneer into
a material tougher in proportion to its
weight than anything engineers had ever
worked with.
NOT alone has the chemist pulled the |
throttle wide open in his own industry.
He has stepped into nearly every other in-
dustry with aids to faster and more efficient
production. The dry-cleaning fluids that
used to clean your best suit are going to
war plants now instead. Theyre used to |
clean metal surfaces—speeding up plating,
lacquering, and painting of those surfaces, |
With new solvents and a continuous-belt |
device, chemical engineers have reduced the
time needed to bleach cloth for uniforms |
from 12 hours to two. And now comes the |
explosive rivet—one of the most fascinating |
little gadgets ever conceived by the mind |
of man—putting an end to one of the most
serious bottlenecks of plane production with
a bang!
Of 250,000 rivets in a Flying Fortress, as
many as 10,000 may be in awkward places
that can be reached from only one side.
Today these are fastened with a rivet that
has a small charge of explosive in its shank.
When the rivet is in place, the charge is set |
off by heat from an electric gun. Nothing |
crude about that operation! Chemistry con-
trols the force of that tiny explosion to
within 1/20,000 of an inch! The chemist
even plays his part in easing the vital war-
time shipping problem. Through working
out ways of dehydrating foods without de-
stroying flavor or value, he makes one ship
do the work of four in carrying foodstuffs
to our allies.
When peace comes, the chemist will slip
back into civilian clothes as easily as he
slipped into uniform on December 7. Even
now he’s doing things for civilians every
day.
Don't let anyone tell you that, because we
can no longer get Asiatic pyrethrum for in-
secticides, you'll be eaten alive by mosqui-
toes and gnats when warm weather rolls
around again. Chemists have discovered a
more deadly insect Killer in the thiocy-
anoacetate of a secondary terpene alcohol,
which comes from the pine trees of our
own South. And if that should fail to im-
press your particular brand of houseflies
or other pests, they've found a homegrown
source of rotenone in a weed that grows in
Texas.
Though you can't get copper screening
for your windows any more, your chemist
will ‘see you through. He's perfected win-
dow screens without wire—woven of plastic
—and your hardware man will probably
have them by next summer. If military
needs should call for all the leather we can
produce, shoes without leather are ready
to fill in the gap.
You can’t get chamois skin any more, so
how are you going to keep the windows
clean? Well, your chemist has found the
answer even to that. After just two hours
in a new tanning solution of chromic sul-
phate, sulphuric acid, and sodium silicate,
the skin of a plain American sheep turns
out to be the best chamois cloth you ever
saw!
Does the front porch need painting? Well,
by next year your paint worries should be
over. There are new pigments to take the
place of the zinc needed in war industry;
new synthetic resins to double for the nat-
ural resins we used to get from Zanzibar,
the Congo, and Borneo. And new vegeta:
ble oils.
Our paint industry was hard hit when Jap
armies swarmed down on the Burma Road,
cutting off our biggest source of imported
tung oil. War had already dried up the
stream of other imported oils needed for
the making of paints. But now a chemist
has come forward with news that ought to
make headlines but will not, because we've
grown to take miracles for granted. Start-
ing with our homegrown corn, soybean, or
linseed oils, chemists can give us new oils
tailor-made to fit any requirements.
The postwar world will be one of abun-
dance. The word “scarcity” will have lost
its meaning. There will be enough fertilizer
nitrogen to turn every farm into the equiv-
alent of a tropical garden. Out of new ma-
terials already developed we shall build
homes lighter, airier, better insulated and
temperature-controlled, at a fraction of the
cost and effort that home-building used to
involve. The whole
process of produc-
ing, storing and ship-
ping the things we
eat, wear, and use
will be vastly
changed for the bet-
ter.
Any preview of
that world would be
fanciful and distort-
ed. But you can see
its beginnings if you
watch what chem-
ists are accomplish-
ing in a score of
fields that are going
to bring a revolution
in our ways of living.
-
Contributor (Dublin Core)
-
Alfred H. Sinks (Article Writer)
-
Language (Dublin Core)
-
eng
-
Date Issued (Dublin Core)
-
1943-02
-
pages (Bibliographic Ontology)
-
58-63
-
Rights (Dublin Core)
-
Public Domain (Google Digitized)
-
Archived by (Dublin Core)
-
Matteo Ridolfi
-
Marco Bortolami (editor)
 Popular Science Monthly, v. 142, n. 2, 1943
Popular Science Monthly, v. 142, n. 2, 1943