-
Title (Dublin Core)
-
Blending Metals to Arm Our Fighting Men
-
Article Title and/or Image Caption (Dublin Core)
-
Blending Metals to Arm Our Fighting Men. Alchemists of the Melting Pot Make the Material Fit the Job
-
extracted text (Extract Text)
-
IMAGINE an exnaust valve for an airplane
engine fashioned to exact size and com-
pletely finished in one operation. Its rim
consists of an alloy especially suited to
holding an accurate seating surface. Its
cap merges over into another alloy, adapted
to resist high temperature. And the stem,
made of a third alloy, resists wear as it
moves back and forth through its guides.
Here is just suck a problem as a designer
may turn over to a metallurgist to solve. It
won't be an easy one. For one thing, the
alloys must expand and contract at the
same rate, or strains may break the piece.
But producing such tailor-made combina-
tions, where none are already known, is the
metallurgist's job. From what has been
called the Iron Age, we have progressed
into the Age of Alloys. Within recent years,
it is said, no less than 10,000 new blends
of metals have been discovered.
Probably the most out-of-the-ordinary al-
loy, employed in radioactive spark plugs
currently available for passenger cars and
motor trucks, has been developed by the
Firestone Tire and Rubber Company. Nickel
ard a minute quantity of polonium, a mem-
ber of the radium family, serve as the ingre-
dients. Drawn into wire, the odd mixture
forms the points of the plug. Since the
polonium ionizes the intervening particles
of gas, or makes them conductors of elec-
tricity, a spark jumps the gap with ease.
This favors quick starting, even in cold
weather and with a low battery. Also, the
catalytic or reaction-promoting effect of the
special points is said to aid combustion, giv-
ing increased power and fuel economy. De-
spite the fact that increasing pressure ordi-
narily shortens the distance an electric
spark can leap, the radioactive plugs are
reported to operate reliably under extremely
high compression—a quality that may help
to improve the efficiency of engines for to-
morrow's cars. In contrast with other ele-
ments born of radium, polonium emits only
harmless rays, and the plugs may be han-
dled, pocketed, or worked over in complete
safety.
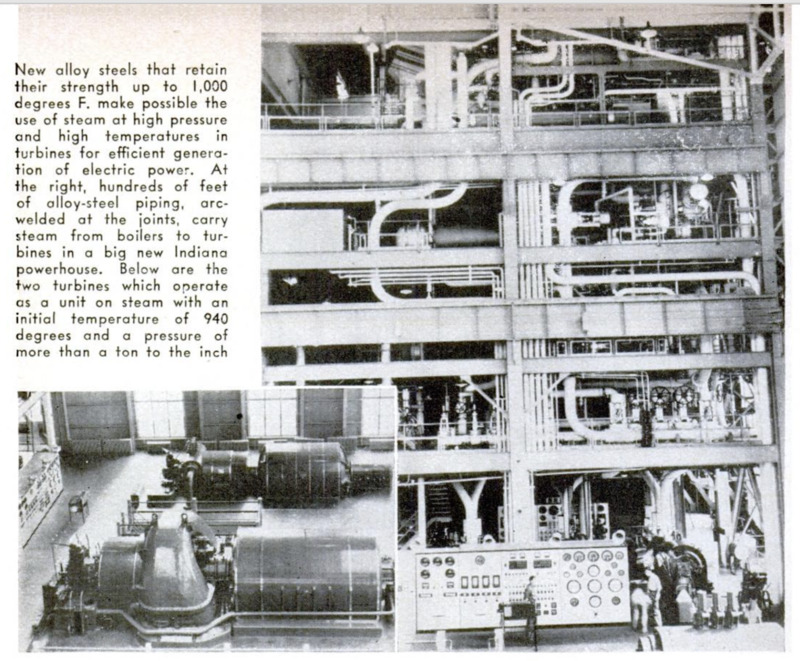
Whirling vanes grow red-hot under the
constantly rising temperature and pressure
of steam fed to modern turbines. The blades
must fit within exacting tolerances for effi-
clency, and resist any tendency to “creep”
or yield to terrific heat and strain. Alloy
steels containing molybdenum, tungsten, or
vanadium maintain Food strength up to
about 1,000 degrees F. But this safety limit
now is being crowded. Operating at the rec-
ord thermal efficiency of 33.5 percent, from
coal to electricity, a big new Indiana power
station consumes steam at an initial tempera-
ture of 940 degrees and a pressure of more
than a ton to the square inch. Since there
is no reason to believe that this is a limit,
new alloys good up to 1100 degrees are
being developed and will be available after
the war.
Another alloy known as Kovar, originally
developed as a metal sealer for electronic
tubes, has been adapted to measure heat in
plane engines and wings by electrical resis-
tance. When war demands depleted the sup-
ply of a special metal alloy used to make
plane thermometers, Westinghouse metal-
lurgists, by making minute changes in the
ingredients and process of Kovar, arrived at
an alloy that has the required properties of
resistance. Four 13-pound ingots were pro-
duced, each of which has enough Kovar to
make thermometers for 20,000 four-motored
bombers.
Coming closer home, it matters a lot to a
man and his disposition how smooth a shave
he gets from a razor blade. Steel alloyed
with chromium and vanadium makes a
good blade—but these ingredients have be-
come increasingly hard to get. Fortunately,
metallurgists have found that more abun-
dant “mixers” for alloy steel—molybdenum,
manganese, and silicon—serve the purpose
as well, if not better. Similar alloys make
fine chisels and axes.
Other special purposes requiring tailor-
made alloys include bits of hardware to re-
pair human bones; nonsparking tools for
workers in powder mills; plugs of low melt-
ing point for fire-fighting sprinkler sys-
tems; instruments practically unaffected by
temperature for precision measurements;
and apparatus capable of withstanding cor-
rosion of all sorts, from sea spray to pow-
erful acids. By blending metals in the right
proportions, and by suitable heat treatment,
a skilled metalworker obtains just the de-
sired degree of hardness, softness, ductility,
structural strength, machinability, and all
the other factors that suit an alloy to its job.
More than half of the chemical elements
are metals—a goodly assortment to start
with. Just as primeval forces of nature left
some of them in a pure or “native” state, so
natural alloys have been found—for ex-
ample, combinations of silver with gold and
copper. But by far the majority of pure
metals, and likewise of alloys, have been
created by the artifices of man.
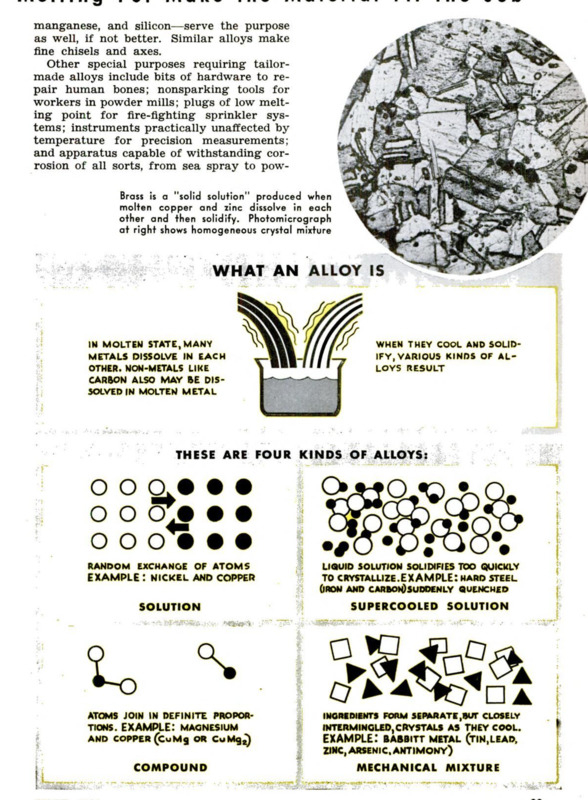
Just what is an alloy? Copper, tin, and
zinc are not alloys, but elementary metals.
Bronze and brass are true alloys, produced
by blending copper with tin or with zinc.
In other words, alloys are hybrid metals.
As used today, the word “alloy” might be
defined as a solid metallic substance con-
sisting of a solution, a chemical compound,
or a reasonably uniform mechanical mixture
of a metal with one or more other metals or
nonmetals. Note that this definition is broad
enough to include ordinary steel. Until
recently, all alloys came from the melting
pot, which yields examples of each type.
Most metals readily dissolve in each other
when liquefied by heat. Let them cool and
solidify, and you are likely to have a “solid
solution”—a crystalline blend in which the
identity of each ingredient has vanished.
Certain pairs, like copper and nickel, form
solid solutions in any proportion. Monel
metal, a corrosion-resisting alloy for kitchen
sinks and industrial purposes, contains about
three parts of copper to seven parts of
nickel. Five-cent pieces formerly were made
of three parts of copper to one of nickel.
To make steel, carbon is dissolved in
molten iron. If this blend cools slowly, the
carbon will form separate granules and the
resulting metal will be soft. But if a white-
hot solution of carbon and iron is “quenched,”
or suddenly chilled, it solidifies too quickly
for the carbon to separate, and the product
is a hard steel like glass, a supercooled
“liquid.” By varying heat treatments, steel
of any desired characteristics may be ob-
tained.
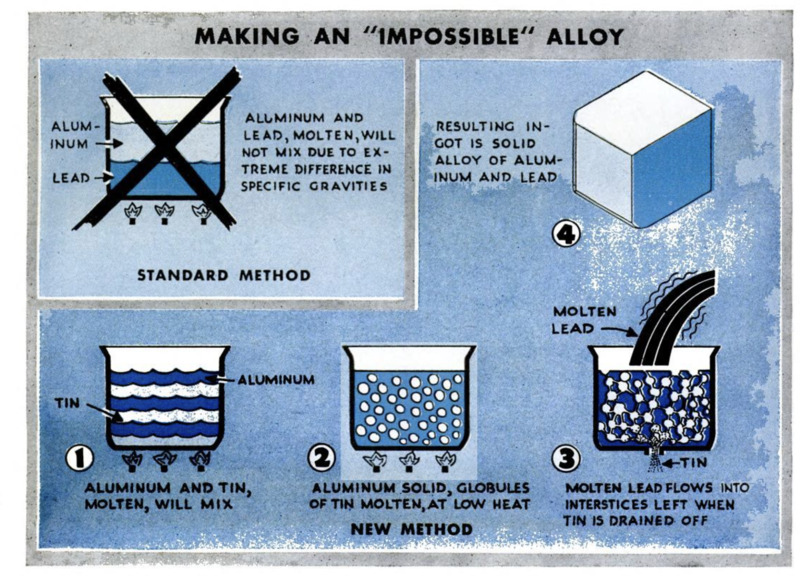
Metals like aluminum and lead cannot be
mixed successfully while molten, because
the lightweight aluminum persistently floats
on top of the heavy lead. Nevertheless, an
indirect method does the trick, according to
a recent report to the American Society for
Metals. To make an aluminum-lead alloy,
the lighter metal first is alloyed with tin.
Upon reheating, the tin melts first, forming
liquid pools amidst a treelike pattern of
solid aluminum. Now molten lead, poured
over the porous mixture, seeps through it
and displaces the tin, which escapes through
a hole at the bottom of the vessel. Like
soft steel, this offers an example
of a nonuniform alloy. Another is
Babbitt metal, used for machine
bearings, which contains a variety
of ingredients. The mechanical
mixture contains hard particles
which take most of the load. As
the bearing wears, these are
pressed back into the softer ma-
trix, so that the surface always
remains smooth.
Some of the alloys that come
from the melting pot are neither
solutions nor mixtures, but definite
chemical compounds. An atom of
copper may permanently combine,
for example, with either one or
two atoms of magnesium, form-
ing compounds represented by the
chemical formulas CuMg and
CuMg:. In this case, the propor-
tions of the combining metals are
absolutely fixed.
More recently, two ways of
making alloys without melting
the ingredients have been dis-
covered. In one of them, called
“powder metallurgy,” pulverized
ingredients are intimately mixed,
and then heated in furnaces at a
temperature below the melting
point, until they coalesce. This
scheme has been applied to make
materials like tungsten carbide, |
which forms extremely hard in-
serta for the cutting nda of ma-
chine tools. Three powders are
used—tungsten, carbon, and me-
tallic cobalt, the last serving as
a binder.
Electrodeposition, akin to elec-
troplating, now offers a way of
making alloys without heat. Its
novelty lies In plating more than
one metal at once. Steel hard-
ware, for instance, may be plated
with brass, from a solution sup-
plying the metallic ingredients of
the alloy. Electric outlet boxes
are protected from rust by de-
positing a zinc-cadmium alloy
upon them. Some modern bright
nickel plating owes its brilliance
to the fact that chromium has
been used along with the nickel.
While many such alloys can be
made by furnace processes, the
electrodeposition method 1s much
simpler and cheaper.
Made into alloys, metals often
take on entirely new properties.
Tests show that adding a teacup
of silver toa ton of copper makes
an alloy that conducts electricity
just as well, and is twice as heat-resistant,
as plain copper. Aluminum and gallium, a
rare metal soft enough to be cut with a
knife, make a bizarre combination. Alumi-
num does not melt below 1,200 degrees F.,
nor does gallium below 85 degrees. Melt
the metals together, however, and the alloy
will stay liquid after it has cooled! Con-
versely, mercury, the only liquid metallic
clement, forms a hard and permanent
amalgam, or alloy, when it is mixed with
silver or gold to fill a tooth.
All the skill of the alloy makers has now
been called upon to meet the emergency of
war. Imported luxuries such as tin, chromi-
um, and tungsten can be used only sparingly,
if at all. Consider that a 37-mm. antiair-
craft gun uses up a ton of copper in every
20 minutes of firing, and it becomes plain
why a formerly abundant metal has become
scarce. So has nickel, the alloying in-
gredient of guns and of armor plate. Mili-
tary needs tax the mines of the United
States, one of the world’s richest countries
in natural resources, for every metal—with
the notable exception of lead, of which we
have enough, and of a few minor elements.
Therefore lead alloys, serviceable in thin-
ner sheets than pure lead, today vie with
copper for roofing and flashing. Indium, an
unfamiliar but plentiful domestic metal, now
makes a valued ingredient of bearing alloys.
“Tinless bronze,” containing silicon, replaces
a desperately needed metal with the most
abundant element, next to oxygen, on earth. |
Silver solder, containing lithium metal, has
been found a superior material for brazing
tungsten-copper electrical contacts. |
By far the greatest innovations, however,
have taken place in the kingdom of steel.
Available alloy ingredients, and war needs,
both have been shifting so erratically that |
mills have been fortunate to keep just a |
jump ahead. No sooner was a threatened
manganese shortage averted than vanadium,
chromium, and even home-mined molybde-
num took their turns on the ration list.
Metallurgists had to juggle their alloy
formulas to suit.
“Lean” alloy steels, also known as na-
tional emergency or “NE" steels, have now
come to the rescue. By urgent Government
request, metallurgists have developed a
series of steel formulas that pare to the
limit the use of scarce materials formerly |
often employed in wasteful quantities. The
result is said to equal or excel previous
metals for all except a few special purposes.
At least half of all the alloy steel being
made today is NE steel, and thousands of
tons of other ingredients are being con-
served for uses where they are indispensable.
These difficulties, however, have not kept
the United States from reaching a steel-
production rate of 89 million tons a year—
a little less than twice the combined pro-
duction of the Axis countries.
Steels containing small amounts of alloy-
ing metals are finding uses undreamed of a
year ago. One of the most outstanding is to
be found in aviation, where a steel with less
than two percent of alloy metals has been
substituted for aluminum alloy in the con-
struction of combat training planes. This
substitution has made available large quan-
tities of aluminum for heavy-bomber con-
struction where the saving of every pound
of weight means that just that much more
fuel and bomb load can be carried.
During the first World War, the Germans
were said to have replaced brass with steel
in making cartridge cases. When shortages
of copper and zinc began to occur in this
country, people asked, “Why don't we do as
the Germans did?” Investigation showed
that while the Germans had made the re-
placement, they had done so with only
limited success. For barrage action and
other uses, steel cases were found to be a
failure. In the intervening years no nation
was able to solve the problem of how to
make steel cases for all guns—until last
summer. Details can not be given for ob-
vious reasons, but we can now boast that
our Army metallurgists have done the im-
possible—for today an amazing torrent of
steel shell cases is pouring out of this coun-
try into the various theaters of war all over
the world.
Cast iron, too, has profited from recent
research. Probably the last quality a lay-
man would expect in cast iron would be for
it to be flexible, rather than brittle. Yet an
improved form, called Meehanite Metal, is
just that. Crankshafts made of. it supple-
ment the use of forged steel in heavy-duty
Diesel engines. To convince skeptics, a
block of the metal has been cut to form a
coil spring about 10 inches long and two
inches in diameter. It can be stretched or
bent double, returning each time to its
original shape.
After the war is won, alloys released from
martial tasks will transform the objects
of everyday life. The lightweight metals,
aluminum and magnesium, will be more
plentiful than ever before. Their alloys,
now reserved for building warplanes, will
then be able to compete in a big way with
other structural metals. Combinations of
little-known metals, worked out to fill im-
perative war needs, will challenge the
dominance of long-accepted materials in all
civilian fields. And many a newly devised
alloy, shelved for the duration because of
prior wartime needs for its ingredients, will
come out of hiding to enrich our supply of
materials for beauty and utility.
-
Language (Dublin Core)
-
eng
-
Date Issued (Dublin Core)
-
1943-06
-
pages (Bibliographic Ontology)
-
98-103
-
Rights (Dublin Core)
-
Public Domain (Google digitized)
-
Archived by (Dublin Core)
-
Matteo Ridolfi
-
Alberto Bordignon (Supervisor)
 Popular Science Monthly, v. 142, n. 6, 1943
Popular Science Monthly, v. 142, n. 6, 1943