-
Title (Dublin Core)
-
Synthetic Ammonia Makers
-
Article Title and/or Image Caption (Dublin Core)
-
Snatching the Ingredients of Victory from Air and Water. Our Ammonia Makers Perform the Incredible Feat of Taking Something from Nothing to Give Us an Unlimited Supply of Explosives and Fertilizers
-
extracted text (Extract Text)
-
EVERY time a 16-inch gun is fired, 120
pounds of nitrogen goes back into
the air from which it came. Every bul-
let, shell, and bomb dropped on Japs or
Nazis comes into being only with the
aid of ammonia, a simple compound
made up of three parts of hydrogen and
one of nitrogen. Yet, if we had to fight
this war with only the facilities for
making ammonia that we had during
World War I, we should be licked before
we started.
The difference between 1918 and to-
day is that now the United States has
a highly developed industry capable of pro-
ducing synthetic ammonia—and the hun-
dreds of other war chemicals derived from
ammonia—using only air and water as raw
materials. In the last war, when all coun-
tries relied upon natural nitrates imported
from Chile for explosives and other nitrogen
chemicals, this country and its allies were
lucky. The Germans tried to cut off British
supplies from Chile but, after the naval bat-
tles of Coronel and the Falkland Islands,
they themselves were blockaded without the
vital nitrates. In fact, if they had not cap-
tured seven ships loaded with Chilean ni-
trates at Antwerp, they would probably
have lost the war by 1916. This lucky cap-
ture gave them a breathing spell while they
built a plant to use a new process developed
by Prof. Fritz Haber—a process which freed
them from the need of anything beyond air
as a source of nitrogen, water as a source
of hydrogen, and coal as a source of power.
During that war, only experimental plants
were constructed in this country, for while
the process was theoretically not too diffi-
cult, the trick was to discover and develop
a catalyst that would hasten the union of
nitrogen and hydrogen into ammonia. Only
after six years of Government-sponsored
research did we work out modifications of
the Haber process. But when our scientists
finally came up with a solution, their process
was many times more efficient than the
original German development. It made syn-
thetic ammonia so cheap that, within a few
years, the annual consumption of ammonia
and ammonia derivatives rose manifoldly.
Ammonia prices dropped so low that hun-
dreds of new uses became practicable, par-
ticularly the important one—in war as in
peace—of making fertilizers,
Today, in many commercial plants de-
veloped since 1925 and in a number of plants
specially built for war work, the ammonia
industry is working all-out for munitions
production. Some of our 2,000-odd peace-
time uses have had to be curtailed to make
way for explosives production, but our ca-
pacity for this vital chemical is now so
large that all really essential needs can still
be met.
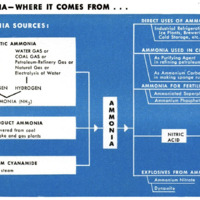
The basic war use for ammonia is that
of providing the foundation for explosives.
One process converts ammonia into nitric
acid, essential in the production of nitrocel-
lulose or guncotton. Nitroglycerin, TNT,
pieric acid, smokeless powder, and ammo-
nium nitrate are all likewise dependent upon
ammonia.
But synthetic ammonia is vital in other
ways to our war machine. It is used for
nitriding or casehardening steels for vital
parts of armor plate, airplanes, tanks, and
guns. Ammonia gas is passed over steel
objects at a temperature of from 900 to
1,000 degrees F. in closed chambers. The
nitrogen from the ammonia combines with
the iron on the surface of the metal to form
an extremely hard case over the inner, softer
core. The interior metal retains its tough-
ness and thus the qualities of two kinds of
steel are combined in a single piece.
Another ammonia compound, ammonium
chloride, is an essential ingredient of the
substances used in many of the smoke boxes,
grenades, and candles that set up smoke
screens. These mixtures, when ignited, pro-
duce a light gray smoke of high obscuring
power. A smoke box dropped behind a de-
stroyer will burn for nine minutes or longer
—long enough for a safe getaway or a ma-
neuver to a new position from which a
counterfire can be started. An accompany-
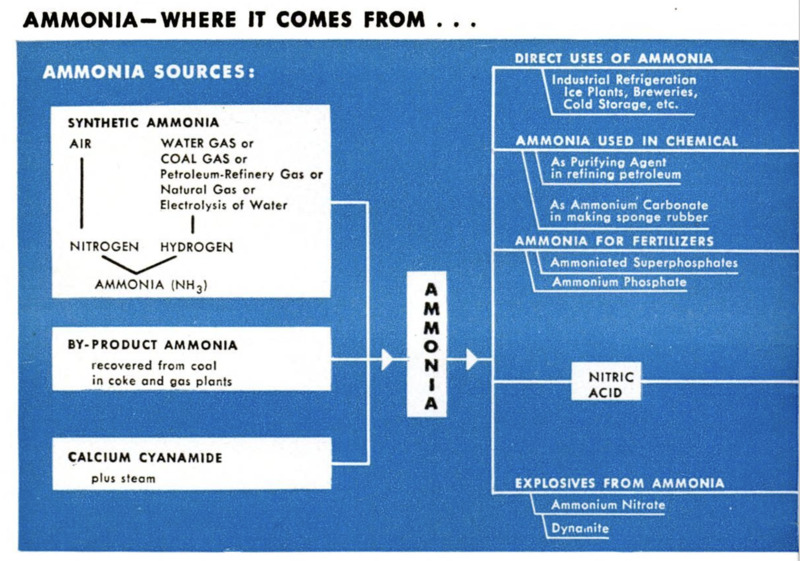
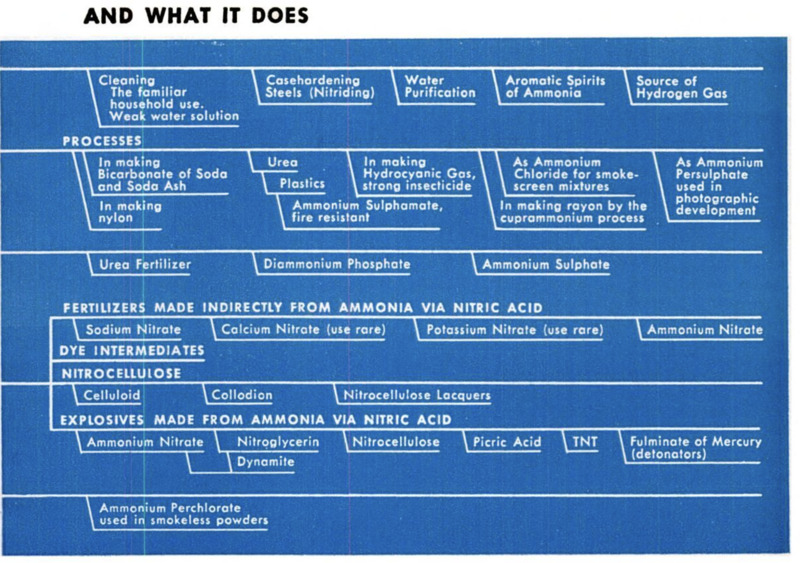
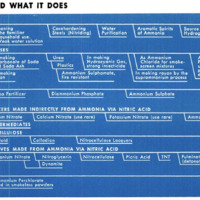
ing chart shows many of the more important
uses for ammonia, in both peace and war.
The synthesis of ammonia is not a com-
plicated reaction. The difficulty is to hasten
the reaction, for at ordinary pressures and
temperatures, nitrogen and hydrogen just
won't combine. Long ago, chemists discov-
ered that when ammonia gas passed through
a red-hot iron tube, it decomposed into hy-
drogen and nitrogen. But a close study
showed that a trace of ammonia always
remained. Then chemists tried reversing the
process. They forced nitrogen and hydrogen
through a hot tube. Again they got a trace—
but only a trace—of ammonia. They con-
cluded that, at normal temperatures and
pressures, the reaction came into equilibri-
um; no matter where you started, you ended
with a little bit of ammonia and a lot of
nitrogen and hydrogen.
Next they tried the same trick under pres-
sure. When they got up to 150 pounds
pressure and 400 degrees C., the equilibrium
point rose; they got nearly four percent of
ammonia. At 50 atmospheres (735 pounds
to the square inch) they could get 15 per-
cent of ammonia. But the reaction was very
slow unless they added a catalyst. Haber's
great discovery was that iron obtained from
iron-oxide granules speeded up the reaction
50 that it could be run commercially. All the
changes since that essential discovery have
concerned three things: improving the cat-
alyst, developing equipment for the use of
higher pressures, and developing a cheaper
supply of hydrogen and nitrogen. Today, half
a dozen processes are in-use. Some rely on
hydrogen obtained by breaking up water by
electrolysis. Others use coke-oven gas, or
“water gas,” the common illuminating or
cooking gas, as a source of hydrogen,
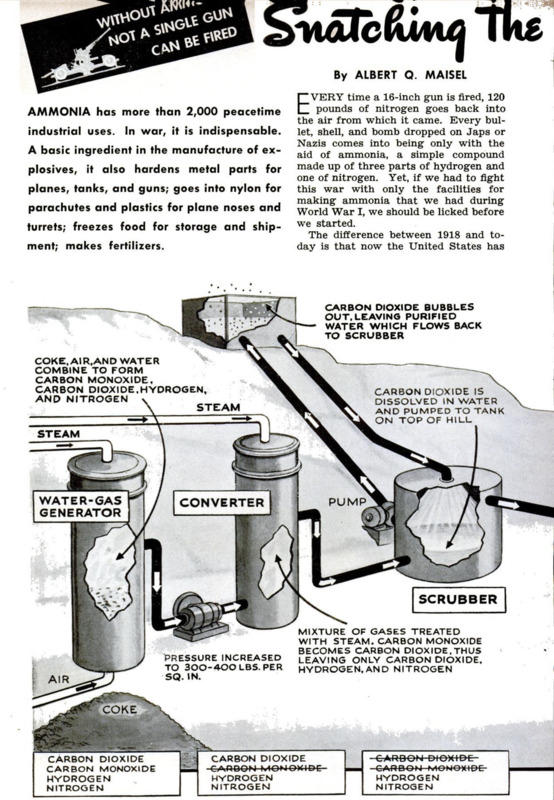
At one of the greatest of our ammonia
plants, operated by the Du Pont Company
in West Virginia, coke is first prepared from
coal in coke ovens. Then it is placed in a
converter, and air and steam are alternately
introduced. Four gases are thus formed. The
steam combines with the carbon of the coke
to form carbon monoxide and hydrogen.
The oxygen of the air likewise combines
with the coke-carbon to form carbon diox-
ide. This leaves the nitrogen of the air as a
fourth gas in the mixture.
Since the four gases are intermixed in
this first stage of the process, the problem
is to separate the carbon monoxide and car-
bon dioxide, which are not needed for
ammonia production, from the essential
hydrogen and nitrogen. The other gases are.
however, used in the making of other prod-
ucts. A little sulphur also is present in the
coke and carries over in the gas mixture
This must be removed so that it will not
contaminate and ruin the catalyst later on.
By an ingenious process the sulphur not only
is withdrawn but is obtained as a very finely
divided paste, especially useful as a fungi-
cide
To remove the carbon monoxide, the pres-
sure is stepped up to 300 or 400 pounds per
square inch and the mixture is treated with
steam in the presence of a catalyst in a
converting chamber, The carbon monoxide
picks up an extra atom of oxygen from the
water and becomes carbon dioxide, liber-
ating more hydrogen. Instead of four gases,
there are now three. L
Carbon dioxide is removed through the
use of a novel system possible because the }
plant is in a deep valley alongside a steep 1
mountain. The carbon dioxide is dissolved 1
in water under pressure, and the water is |
then pumped to a tank on top of the hill.
Here, at atmospheric pressure, the carbon
dioxide bubbles out of solution just as it I
does out of ordinary soda water when the k
pressure is released. The purified water then «
flows down the mountain and back to the
scrubber, where it picks up more carbon
dioxide. ‘The pressure is maintained at the J
scrubber, at the bottom of the hill, by the
hydraulic head of water. Thus only a mini-
mum of power is needed to circulate the
water through the system.
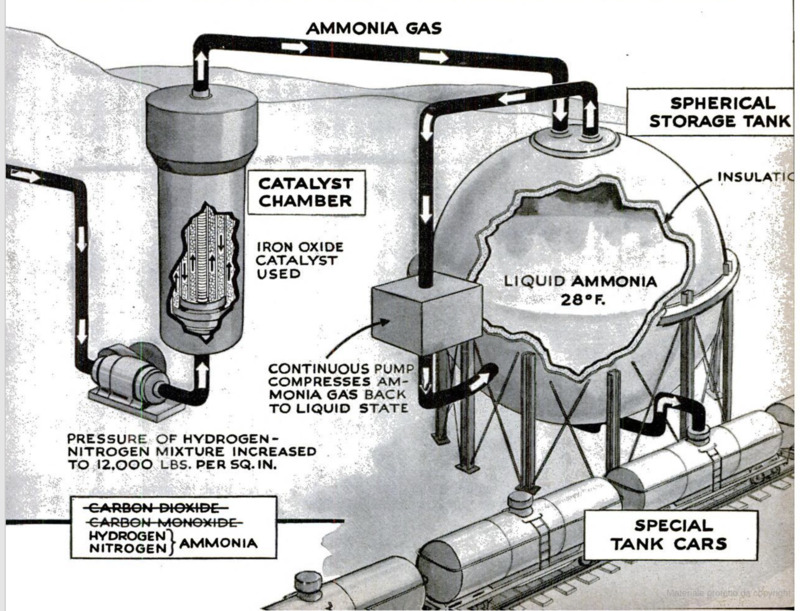
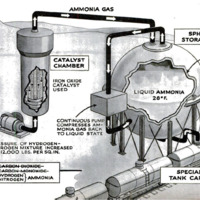
Now, at last, the actual ammonia-making
step is ready to begin. The mixed gases are
first compressed to about 10,000 or 12,000
pounds per square inch. They are then
forced into a converter made of special alloy
steel and capable of withstanding the un-
usual combination of heat (500 degrees C.)
and pressure. Within the converter, the
catalyst is piled over a series of bafies so
arranged that the gas must take a long.
route through the material. This insures
the highest possible degree of conversion in
the shortest time. The catalyst consists of
granules of reduced iron oxide with a small
‘admixture of other materials, such as alumi-
‘num oxide, called “promoters” because they
increase the activity of the catalyst. The
first hydrogen that passes through the con-
verter burns away the iron oxide, leaving
pure iron granules. These become extremely
porous, thus providing a maximum surface
Tor contact with the gases.
This catalytic converter is the heart of
the synthetic-ammonia process. Into it go
hydrogen and nitrogen in proper propor-
tions. Out of it comes ammonia gas, which
can be cooled and compressed to liquid am-
mona. At the Du Pont plant, the ammonia
does its own cooling. As it comes out of
the converter, it is piped to a large, insulated
spherical tank. A continuous pump takes
the ammonia vapor from the top of this
tank and compresses it back into the liquid
state, after which it is again forced back
into the sphere. Under these conditions, the
liquid ammonia is maintained at pressures
only a little greater than that of the outside
air. Its temperature, however, is kept near
its normal boiling point, —28 degrees F. For
shipment, ammonia is pumped into specially
constructed, cork-insulated tank cars.
The most familiar uses of ammonia con-
sume only a small fraction of the total an-
nual production. Spirits of ammonia in the
medicine chest and ammonia dissolved in
water for cleaning purposes are the most
common everyday uses. Ice plants, brew-
eries, cold-storage houses, and industrial
plants use ammonia for refrigeration. House-
hold refrigerators commonly use other lig-
uids, which may not always be as efficient
but Which operate at lower pressures and
are safer in case of a break in the coils.
Along with chlorine, ammonia is used by
‘many cities for water purification, the com-
bination having proved more effective than
chlorine alone.
One compound of ammonia, ammonium
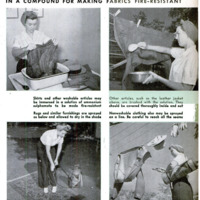
AMMONIA TAKES NEW ROLE
OUR clothing, draperies, upholstery
fabrics, and bed coverings can now be
‘made fire-resistant and safe through the
use of a new compound of ammonia—
‘ammonium sulphamate—one of the latest
developments of this important chemical.
Treatment is as easy as washing clothes,
and the fire-retardant solution can be
made simply by dissolving ammonium
sulphamate (not to be confused with the
sulphate) in plain water at the rate of
one pound in one gallon.
Workers in war industries may treat
their clothing, from coveralls and hats to
‘shoes and socks, with the solution and be
prepared for emergencies. In the home,
the treatment can extend to children's
and adults’ clothing, blankets, linens,
Kitchen towels, ironing-board covers, all
‘upbolsteries, and both heavy and fimy
drapes. Although materials so treated
may be damaged by fire, they are pro-
tected against bursting into flame and
spreading a blaze.
“Articles to be treated should first be
‘washed or otherwise cleaned and then re-
paired. For a war worker who may be
‘exposed to flames in the course of a day's
work, some alterations might be made
profitably, especially in coveralls, Which
should not have cuffs, outside hems, or
open pockets that could catch fiying em-
Bers. Wash goods are soaked in the am-
‘monium sulphamate solution until sat-
‘rated, with a wetting agent such as soap
or a soapless cleanser added to help pene-
tration if the material is new. The articles
are then dried, and pressed if desired, like
ordinary washing.
‘Nonwashable fabrics are hung on a
clothesline and sprayed or brushed thor-
‘oughly. They should remain hanging until
ary. Gloves, shoes, hats, and the like may
be dipped in the solution, and should be
dried in the shade, away from heat.
carbonate, is used for making the sponge-
rubber cushions once used for autos and now
employed for a number of military purposes.
The heat of vulcanization turns this chem-
ical into ammonia gas and carbon dioxide
gas, inflating the rubber into a spongy, por-
ous mass and causing it to rise and fill the
mold.
A strange use of ammonia is as a source
of hydrogen. It may seem odd that, after
all the trouble the ammonia makers take to
get hydrogen into their synthetic ammonia,
they should turn around and offer the prod-
uce as a source of hydrogen. The answer
lies in the ease with which it can be trans-
ported. One standard, 100-pound cylinder of
ammonia yields 3,400 cubic feet of hydrogen,
which would require 17 cylinders if it were
to be shipped in the ordinary way.
More important than any of these uses,
except the war uses today, is the conversion
of ammonia into fertilizers. Synthetic am-
monia has materially reduced the cost of
fertilizers while encouraging a vast increase
in the amount used by American farmers.
Many of the new synthetic materials em-
ploy ammonia directly or indirectly, partic-
ularly the urea plastics, nylon and Lucite.
One of the newest uses for ammonia prod-
ucts is described below.
-
Contributor (Dublin Core)
-
Albert Q. Maisel (writer)
-
Language (Dublin Core)
-
eng
-
Date Issued (Dublin Core)
-
1943-07
-
pages (Bibliographic Ontology)
-
98-103
-
Rights (Dublin Core)
-
Public Domain (Google digitized)
-
Archived by (Dublin Core)
-
Matteo Ridolfi
-
Alberto Bordignon (Supervisor)
 Popular Science Monthly, v. 143, n. 1, 1943
Popular Science Monthly, v. 143, n. 1, 1943