-
Titolo
-
Seawater desalting kit for army and navy flyers
-
Article Title and/or Image Caption
-
Title: A drink from the ocean
-
Subtitle: Flyers no longer need suffer the tortures of thirst when forced down at sea. . . .A compact kit employing a chemical precipitant offers a new solution to an age-old problem
-
extracted text
-
THE recent development of a chemical
precipitant that makes sea water as safe
and palatable as the water from your
kitchen tap marks an all-time high in man's
endless struggle with the problem of mid-
ocean thirst. Never before was sea water
purged of its toxic salts by a practical
method employing chemical reaction.
Directly inspired by the needs of flyers
for additional water rations without addi-
tional bulk, the desalting compound is being
packed in a kit which will occupy the same
space as the current G.I. water can in a
‘chute pack.
The statistics are eloquent. An Army
flyer now carries a 11.5-ounce can of water;
a Navy flyer, two cans. Reckoning a pint
per day as a base ration for a man, the
single can will last barely two days, while
two may be stretched to last for three days.
The kit, containing five briquettes, each
capable of desalting a little over a pint,
would give the Army flyer a potential of six
pints; with one kit and one can, the Navy
flyer would have a potential of seven pints
of potable water.
Developed by the Permutit Company, a
leading water-conditioning firm, with the
advice of the Naval Medical Research Insti-
tute, at Bethesda, Md., the desalting process
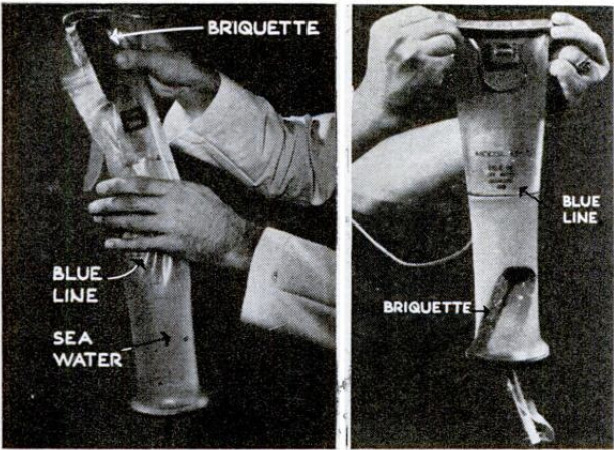
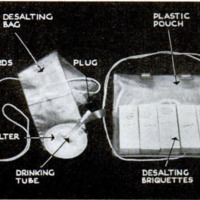
is as simple as making a cup of tea. Packed
in the kit is a plastic drinking bag with a
cloth filter at its base and a drinking tube
set below this. There is also a supply of
chemical, not identified at this time, which
comes in compressed bricks, each the size
of a candy bar. These are guarded from
moisture by two heat-sealed wrappings.
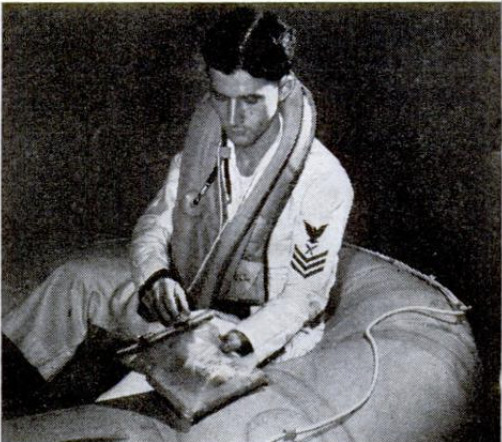
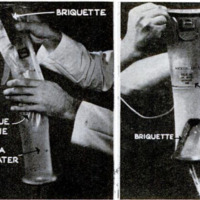
When he wants a drink, the castaway
flyer scoops the bag full of sea water, drops
in a briquette, straps the top opening closed,
and shakes the bag from side to side to
hasten the reaction. In 20 minutes the
liquid is black with tiny granules. Of the
3.5-percent salt volume that sea water holds
(27 percent sodium chloride; 8 percent
salts of calcium, magnesium, and potas-
sium), a total of 3 percent has been pre-
cipitated out.
To take a drink, the survivor simply un-
corks the slim tube beneath the filter and
sucks. The first mouthful will be salty,
from filter leakage, but after that only a
faintly stale taste distinguishes the desalted
liquid from naturally fresh water.
‘Why wasn’t such a simple solution thought
of long ago? Probably because it was never
so urgently needed before. The only hint
of chemical desalting heretofore is a legend
of some plant used by the Phoenicians to
make salt water potable. Beyond this,
mariners seem to have been content to use
distillation methods, or to carry an ample
supply of fresh water if the vessel was too
small for a still. Distillation, the physical
process of drawing off and condensing
vapor from sea water, leaving the salts
and other impurities behind, is still an ex-
cellent method for supplying large groups.
But the weight and bulk of a still preclude
its use on life rafts and small lifeboats,
although there is an experimental miniature
still that may become standard equipment
in larger lifeboats.As with Permutit, the great bulk of de-
salting research has been inspired by the
war-born needs of airmen. One of the morepublicized methods was also a precipitation
scheme, the work of physicist Alexander
Goetz, of California. It involved four steps:
conversion of salts to alkalies, filtration,
precipitation of the alkalies, and a second
filtration. Intrigued by its possibilities, Navy
researchers studied and improved upon the
Goetz process to some extent, reducing the
number of bags used from four to two. At
its simplest, the Goetz method is still more
complex than the Permutit-Navy process. It
has never been officially adopted by any
branch of the service.
Though dissimilar in action, the Goetz and
the Permutit-Navy devices share the advan-
tage of compactness, so that they give a
high ratio of water for their size and
weight. The water cans used by our flyers
weigh a pound each and hold only 11.5 ounces
of liquid. The Permutit kit weighing less
than a pound and a half, can produce five
pints of water. But, like any other chem-
ical method, its efficiency is always lim-
ited by the amount of chemical on hand.
Other processes are just the reverse; the
equipment is heavy and bulky, but it is self-
sufficient. Given salt water, it can keep on
producing fresh water indefinitely. One out-
fit, a compressor refrigerator, is based on
the principle that when brine freezes, its
salts center in “core water” in the middle of
the ice, the ice itself remaining fresh and
saltless. This is in the experimental stage.
Besides the miniature fuel-burning still
mentioned above, two other small stills
have been developed, both ingenious devices
that save the weight of fuel by harnessing a
natural source of heat. One, the “belly still”
draws its heat from the human body. A
hand-cranked vacuum pump keeps pressure
in the “boiler” low enough that water
evaporates at body heat. The resulting
vapor is passed to a vessel trailing in the
sea, which is never warmer than 78 degrees;
the 20-degree drop is sufficient for im-
mediate condensation.
The solar still absorbs heat from the sun
through a square of moist, black toweling.
Evaporation from the wet cloth produces
water vapor, which condenses on the inner
side of a Vinylite covering and drips down to
a tube from which it can be sucked.
-
Autore secondario
-
Jean Ackermann (article writer)
-
Lingua
-
eng
-
Data di rilascio
-
1944-01
-
pagine
-
41-44
-
Diritti
-
Public Domain (Google digitized)
-
Archived by
-
Lorenzo Chinellato
-
Marco Bortolami (editor)