-
Title (Dublin Core)
-
A new discovery shows how to produce dirigibles that cannot catch fire
-
Article Title and/or Image Caption (Dublin Core)
-
Title: Balloons that Can't Catch Fire
-
Subtitle: We discovered how to substitute helium for hydrogen and thus we revoluzionize the dirigible airship
-
extracted text (Extract Text)
-
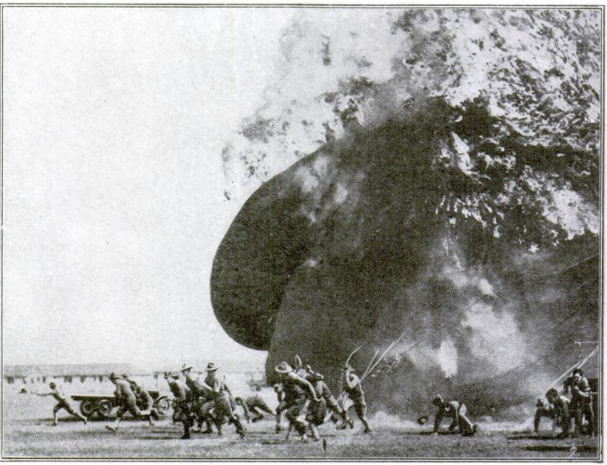
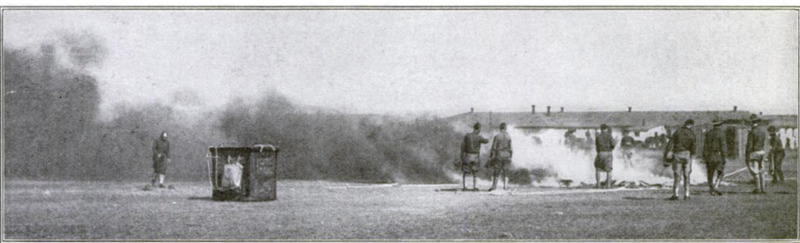
IN a recent test of marksmanship
at one of the army aircraft
schools, two small balloons were
provided as targets for two respec-
tive groups of marksmen. At the
first shot from the first group the
target burst into a mass of flame
and smoke—the effect of an explo.
sive bullet. The second group fired
once, twice, a dozen times. Obser-
vers were sure that some of the
shots had scored hits —but nothing
happened. The firing continued,
with the same negative result.
Something was wrong. The explo-
sive ammunition was pronounced
defective. Report, to this effect
was made formally to the proper
authorities.
Why It Didn't Catch Fire
Only a few officers present knew
the secret. The test was eamou-
flaged, in the sense that the second
balloon could not have been set afire
by any known agency. It contained
the new non-inflammable gas de-
veloped by the government and
guarded as an all-important war secret.
This gas —helium—is now being pro-
duced in large volume. Its value for
peace and war usage is regarded as
tremendous. The story of its develop-
ment from the inception of the idea to
its practical usage has all the elements
of romance of the middle ages.
It might truthfully have been said
to the disappointed army marksmen:
The reason you don’t explode that
balloon is because a Professor Cady,
of the University of Kansas, some ten
years ago made a survey of the gas
and oil resources of that region, and
published a scientific paper, which
happened to be in the files of an old
British scientist in London at the time
one of the Zeppelins was brought
down.
“Suppose the gas would not burn?"
the professor thought. He recalled
having seen the report of an American
professor, illustrated with so-called
iso-helium lines to outline the region
where he thought helium existed. The
British scientist knew, of course, that |
helium was non-inflammable, and light |
enough to lift a balloon or Zeppelin.
He put two and two together in the |
quiet of his study, and wrote a note
to the British Admiralty. That was
the beginning.
Fascination of Helium for Scientists
The Admiralty turned the note over
to the proper scientific branch as & |
matter of routine. Ideas from out-
siders came by the thousands, but this
one attracted attention, chiefly be-
cause helium had a fascination for the |
British scientists. Its characteristic
line had long been a feature of the |
sun's spectrum. But the gas itself |
had never been isolated on the earth.
Tt seemed for a time as if helium |
would never be obtained. Then Ramsay
and Rayleigh got hold of the residue of |
several tons of liquid air that had gone
through the Claude plant in Paris,
and fractionated it, discovering un- |
known constituents of the air (argon,
xenon, krypton) and helium. There
on the spectroscope was flashed the
long-lost helium line! More light was
shed on helium when it was found to
be a constituent of radium emanation.
Radium and helium are elements.
Radium was being transformed into
helium! Were the old - alchemists
right, after all?
Professor Satterlee, of the Universi-
ty of Toronto, made some investiga-
tions that led him to suggest the pos-
sibility of helium’s being the product
of radioactive transformations under
the ground.
English and Americans Collaborate
The Admiralty took the position that,
if there was one chance in a thousand
of getting helium in quantities, it was
worth taking. Professor Satterlee was
requested to consult the American
authorities and to investigate Profes-
sor Cady’s report.
About this time the United States
came into the war, and the British
sent over experts to collaborate with
Colonel Burrill, technical head of our
Chemical Warfare division. He,inturn,
took the matter up with General Squier,
then head of Army Aeronautics, Di-
rector Manning of the Bureau of Mines,
and Admiral Griffin, Chief of the Bureau
of Steam Engineering in the Navy.
Sopn after the matter reached Ad-
miral Griffin, there appeared in his
office a Mr. Carter, who had given up
civilian work to enter the Navy. It
developed that he probably knew
more about the liquefaction of air and
gases than almost anyone else in the
country. Itseemed asif fate had pushed
the right man at the right moment into
Admiral Griffin's office.
The Army and the Navy engaged
companies to liquefy the natural gases
in the areas indicated by Professor
Cady. Congress was asked to appro-
priate money for the production of
argon, It would never do to let the
truth leak out in the midst of a war.
Professor H. N. Davis took hold of
the Army end, Commander Dyer rep-
resented the Navy.
Now there are two big plants pro-
ducing helium in large quantities and
another company about to begin, all
located in Texas. Natural gas is col-
lected and compressed and cooled
down in the same kind of appar-
atus that is used to liquefy air.
As the cooling process continues
everything is liquefied out long
before the helium. The regular
hydrocarbon series, making up ap-
proximately 70 per cent of the
natural gas, liquefies first, and then
the nitrogen, constituting about 30
per cent, is condensed at a temper-
ature of 195° C. below zero. It
requires 268 decrees below ordinary
zero (Centigrade) to liquefy helium,
which is then collected for use in
balloons. The amount of helium in
natural gas is .95 per cent.
Had the Germans Known Helium
In a military sense, helium for
balloons is the answer to the hich-
explosive bullet, just as armor in
the old Navy was the answer to
the guns that riddled wood. Had
the Germans filled their Zeppelins
with helium instead of explosive hy-
drogen, they might not have proved
such failures as city bombers.
The entire art of designing and
equipping dirigibles will be revolu-
tionized. Electrical apparatus,
heretofore barred because of the
ever-present dread of sparks, may
be installed, new types of motors
may be used. ‘A new fie'd is opened
up for development.
Why is there so much helium in
Kansas, Oklahoma, and Tezas? Frob-
ably because of the quantities of radio-
active minerals. Helium, as we have
seen, isa degradation product of radium.
-
Contributor (Dublin Core)
-
Albert Whiting Fox (Article writer)
-
Language (Dublin Core)
-
eng
-
Date Issued (Dublin Core)
-
1919-03
-
pages (Bibliographic Ontology)
-
58-59
-
Rights (Dublin Core)
-
Public domain (Google digitized)
-
Archived by (Dublin Core)
-
Davide Donà
-
Marco Bortolami (editor)
 Popular Science Monthly, v. 94, n. 3, 1919
Popular Science Monthly, v. 94, n. 3, 1919