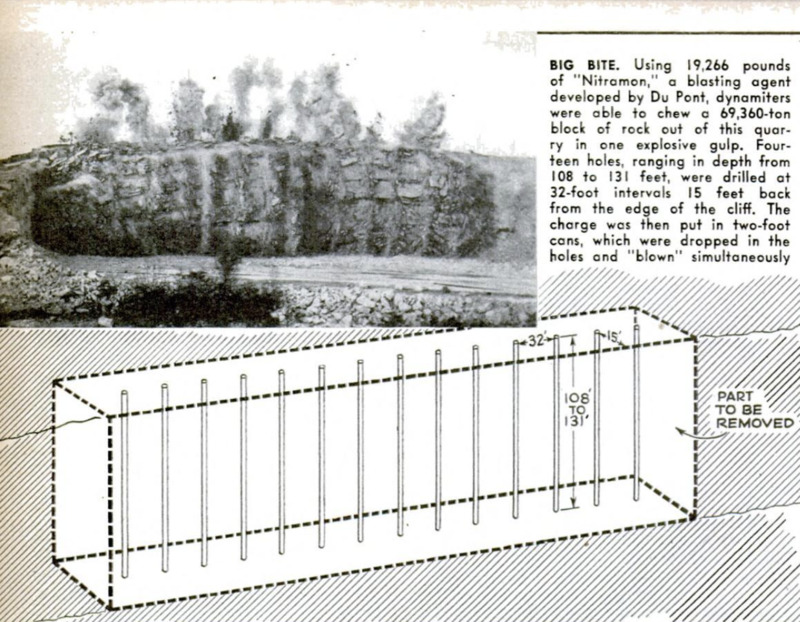
-
Title (Dublin Core)
-
Several kinds of explosives each one with its own particular job in war as in peace
-
Article Title and/or Image Caption (Dublin Core)
-
Title: Teaching explosives to do tricks
-
Subtitle: They're just fat-burning chemicals that pack a punch ... there are many kinds, and each kind has its own particular job to do in war as well as in peace
-
extracted text (Extract Text)
-
MODERN WARFARE is the art of using
explosives to throw explosives. In any
battle, the side which can throw the largest
quantity of explosives the greatest distance
and with the greatest speed usually comes
out the winner.
But what are these things we call ex-
plosives? Most of us think of them as
powerful, violent chemicals which go off
that is, explode—on the least provocation.
Actually, any explosive which meets this
definition is usually too sensitive for war-
time use. The best known of the high ex-
plosives, TNT, is so insensitive that it will
not explode even if hit directly by a high-
powered rifle bullet. As for power, there is
actually more energy in a pound of coal
than in a pound of TNT.
‘The secret of explosives lies in their con-
trol. They must be used in such a manner as
to release their power suddenly, at just the
right second and in just the right place.
With industrial explosives, the right time
means when everyone has moved out of the
danger zone around the explosion, and the
right place may mean that point in a bore
hole which will break away just the desired
amount of rock. With military explosives,
there are even more precise demands as to
time and place, for a shell must not explode
under the impact of firing it from a gun, but
it must explode at a predetermined second.
Actually, explosives are simply compounds
or mixtures which burn. But they differ
from ordinary burning things in two re-
spects; they burn much faster, and most
of them carry their own oxygen supply.
‘When you burn a piece of wood, its outer
surface combines with the oxygen in the
air, and a large part of the wood is trans-
formed into gases. In the reaction a great
deal of heat is released. If you used small
chips of wood, they would burn much faster
because they present more surface to the
air. And if you burned wood powder in
an atmosphere of pure oxygen, combustion
would be even more speedy. In fact, it
would take place so fast that you would
have a miniature explosion.
When you deal with regular explosives,
vou speed up the combustion still further,
because chemicals are present which release
oxygen and thus hasten the process. Most
of these chemicals are compounds of nitro-
gen and oxygen, for nitrogen is a “lone wolf”
element which separates readily from com-
bination with others.
Hundreds of fast-burning, or explosive,
compounds and mixtures are known, but
only a few can be used as military ex-
plosives. These are the ones which remain
inert until the proper moment and then
give off their energy under a controlled
impulse.
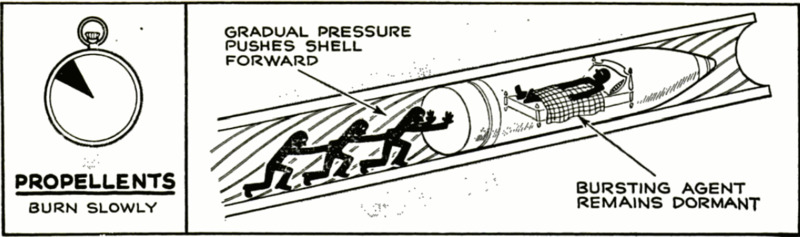
There are two basic types of military
explosives. Those called propellants are
relatively slow-burning and give off large
quantities of gas. When one of these is
exploded within the barrel of a gun, it builds
up its pressure gradually and thus does not
shatter the gun itself. Instead, it sets in
motion the one part of the assembly that
can be moved—the bullet or shell—and, as
the gases expand, this is guided by the gun
barrel and moves out to fly through space
toward the enemy. Of course, the so-called
slow burning of a propellant is slow only in
comparison with the rapid detonation of a
high explosive. Its burning actually occurs
in a fraction of a second.
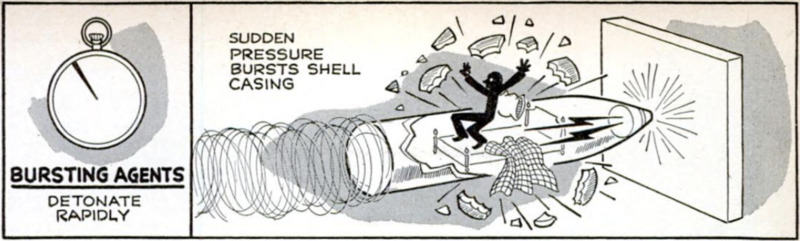
The second great class of military ex-
plosives consists of the bursting agents.
These are insensitive explosives capable of
withstanding the great heat and shock ex-
perienced within the shell when they are
hurled out of the gun barrel. But when they
finally do explode, the reaction takes place
50 fast that they shatter their steel con-
tainers and wreak havoc wherever they land.
While propellants explode by “burning,”
bursting agents explode by a process known
as detonation. The difference is merely one
of speed, but it is a great difference. Ordi-
nary smokeless powder burns in the open
air at a rate of three or four inches per
second. Inside a gun barrel, heat and pres-
sure speed up this rate very greatly. But
TNT detonates at a rate of over 22,000 feet
per second, and nitroglycerin has a detona-
tion speed of over fire miles per second!
Both propellants and bursting agents,
however, are relatively insensitive. This is
an essential quality because otherwise they
would frequently explode in the manufactur-
ing plant, in shipment, or in an ammunition
dump. But in order to cause them to go off
at the proper time, ordnance experts have
had to develop other explosives known as
primers or detonators. These are sensitive
enough to explode from the shock of a ham-
mer blow or the percussion of a firing pin.
On small arms such as shotguns or rifles,
a tiny detonator is sufficient to initiate the
explosion of the smokeless powder in a shell.
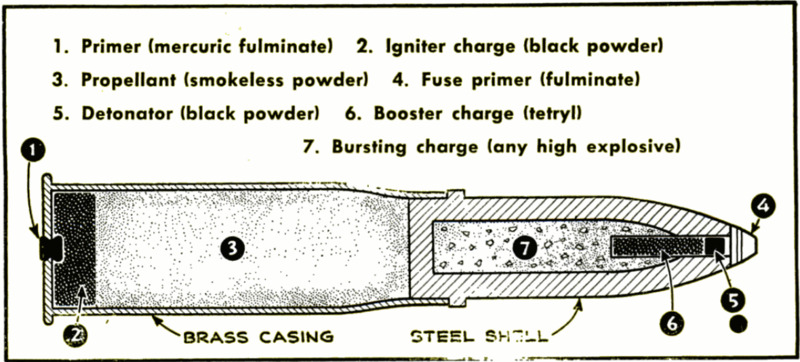
But in larger cannon, a chain of explosions
frequently is necessary. The firing pin of
a big gun will detonate a small and sensitive
primer of fulminate of mercury. This will
in turn ignite a charge of black powder, and
this will pass on the explosion to the less
sensitive smokeless powder that hurls the
shell out of the gun and toward the enemy. |
The same sort of train is used within the |
shell itself. With an armor-piercing shell,
the artilleryman wants to be certain that the
projectile first pierces the enemy's protective
armor and explodes within the fortification
or the hull of a ship. He accomplishes this |
by equipping his shell with a delayed-action
fuse mechanism. The relatively insensitive
explosives within the steel shell actually
withstand the terrific shock of impact upon
armor. But this shock slows up the shell,
and causes a striker set in the back of the
shell to hit a tiny detonating charge. This
in turn may set off a booster charge and,
completing the sequence, the booster will set
off the high-explosive shell. Such a train |
of explosions can be designed to take any |
desired number of fractions of a second, or
even seconds or minutes.
Even though propellants are relatively
slow-burning, ordnance experts find it neces- |
sary to control their rate of burning. The
ideal explosion is one which will continue
with mounting force until the shell has left |
the gun. The pressure within the gun should
build up gradually so as not to shatter the
barrel; and none of the explosive should be
wasted by failure to be consumed before
the bullet has left the gun.
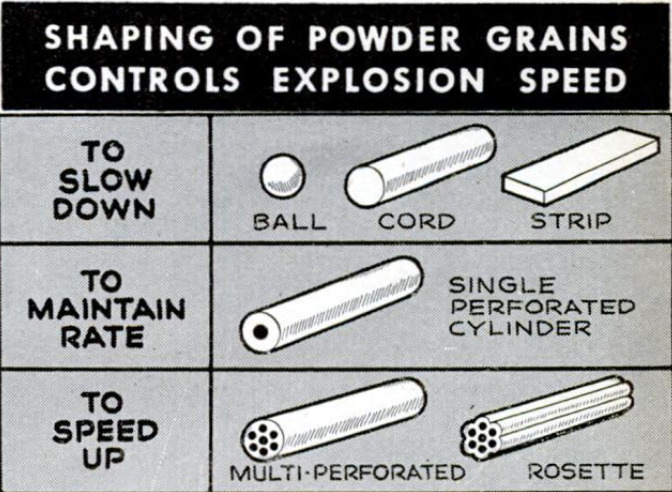
To control the burning of the propellant
powder, it is formed into grains. The larger
the caliber of the gun, the larger the grains
of powder which are used. Increase in size
slows up the burning, since the grains start
burning on their outer surfaces and com-
bustion progresses inward only as the outer
layers are consumed.
This method of slowing up burning pre-
sented the engineers with another problem.
Originally, all grains were formed in spheri-
cal ball shape. Thus, they burnt fastest at
the start of the explosion, when their outside
surfaces had the greatest area. As each
pellet burned away, it became a smaller
sphere and thus produced its gases at a
slower rate. Such round-grain pellets built
up high initial pressures, which quickly
dropped off. This didn't matter very much
with small arms, but it was a serious draw-
back when large guns were involved.
‘To overcome this difficulty, ord-
nance experts have developed a
number of different shapes for
powder grains. The British evolved
cordite, a smokeless powder in long,
cordlike grains. The French de-
veloped thin, flat strips. In Amer-
ica, engineers worked out still an-
other way of regulating the rate
of burning. They continued using
round pellets, but they coated them
with relatively slow-burning outer
surfaces. Thus, when the burning
surface was greatest in area, at
the beginning of the explosion,
these deterrent coatings slowed
down production of gases. Once the
outer surfaces of the sphere were
consumed, the faster-burning inner
powder compensated for the reduc-
tion in the area of the burning sur-
face. Such a powder grain would
be termed “balanced,” in that it
produced its gases at a fairly uni-
form rate,
But American engineers have
gone even further and developed “progres-
sive-burning” powder grains—forms that
increase their rate of burning as they are
consumed. This is accomplished by forming
the powder into cylinders with as many as
seven holes running through a cylinder,
parallel to its axis. While the outer surface
of the cylinder decreases in area as it burns,
the surfaces of the holes increase. Because
they are designed to more than compensate
for the decreased area of the outer surface,
they actually produce gases at an accel-
erated speed toward the last quarter of
their explosion.
With such powders, it has become possible
to design guns of high efficiency. Gun bar-
rels can be thinner than before, and still
withstand the pressures in the barrel, for
these pressures build up gradually and the
power of the explosion is not wasted in
“flash” after the shell has left the gun.
American engineers were also responsible
for the development of smokeless powders,
although these were first adopted by other
countries. Black powder, which had been
used from the earliest days until after the
Spanish-American War, had a number. of
drawbacks, not the least of which was the
fact that it gave off large quantities of
smoke which aided the enemy in locating
artillery.
Smokeless powders were made possible by
the development, toward the end of the nine-
teenth century, of nitrocellulose. They are
more stable, eliminating the greater part of
the muzzle flash and smoke formerly asso-
ciated with all firing pieces. However, they
are not completely smokeless, although
their small white puff is far less easily lo-
cated than the dense black smoke of the
powders formerly used.
Most armor-piercing shells depend ‘upon
steel rather than upon explosives to secure
their armor-piercing effect. Such shells are
made of extremely hard, tough, and heavy
steel, hurled at great velocity from long-
barreled guns. Large armor-piercing shells,
such as those designed to penetrate battle-
ship armor, are almost solid chunks of metal.
The weight of their high-explosive charge
can be only five or six percent of their total
weight. Yet, that five or six percent is
sufficient to cause terrific damage within
the close confines of an armor-plated hull
once the steel slug has penetrated.
Another method of armor piercing, which
has not yet been adopted by any warring
nation—except possibly in secrecy—involves
the use of what is known as the Munroe
effect. This phenomenon was first dis-
covered by Charles E. Munroe, professor of
chemistry at Columbian University, Wash-
ington, D. C., and was announced nearly 45
years ago in POPULAR SCIENCE MONTHLY.
Professor Munroe, in conducting his ex-
periments at the Naval Torpedo Station in
1888, noted that explosive waves tended in
certain cases to reinforce each other. His
discovery was made quite by accident.
Professor Munroe used to mark blocks of
molded guncotton for identification by coun-
tersinking letters into the surface of the
blocks. When such blocks were laid upon a
steel plate and exploded, Dr. Munroe noted
that after the guncotton had detonated, the
letters were reproduced upon the iron plate.
What was most singular was that when
the letters on the guncotton were raised
above the surface they also came out raised
on the iron plate.
“In experimental investigation,” Dr. Mun-
roe wrote in POPULAR SCIENCE, “I eventually |
bored holes of various diameters and depths
in guncotton cylinders and in the last in-
stance, I bored a vertical hole completely
through the cylinder. These cylinders were
each placed on a similar iron plate. When
they were successively fired, it was found
that the deeper and wider the hole in the
guncotton was, the deeper and wider were
the holes produced in the iron plate. When
the completely perforated guncotton cylin-
der was fired, the iron plate was found to be
completely perforated.”
‘What Munroe had done was to shape an
explosive charge in such a way as to cause
the detonation waves to reinforce each other.
He concentrated much of the explosive force |
in one direction, parallel to the axis of the
cylinder. |
Conceivably, such shaped charges might
be used for armor-piercing purposes on
large shells, eliminating the necessity of
sacrificing ninety percent or more of an
armor-plercing shell to the weight of steel.
A shaped-charge shell would be almost en-
tirely made up of explosives, with only a
thin outer container of metal. Gunners using |
such a shell could deliver a far greater
quantity of explosives to a point of impact.
So-called industrial explosives—of which
dynamite is the best known and most widely
used—play an important part in the winning
of the war, both by making production
possible at home and in their application
on and immediately behind the battlefields.
Dozens of new and improved methods of
using such explosives have arisen directly
out of the war effort.
Dynamites have changed greatly from the
first product developed by the great Alfred
Nobel. Nobel made dynamite by absorbing |
highly explosive nitroglycerin in an inert |
earth called kieselguhr. This made a power-
ful explosive which was still sufficiently
insensitive to shock to permit its being |
transported and stored without great danger.
Today, instead of kieselguhr, dynamite
makers use mixtures of wood pulp, nitrate |
of soda, nitrate of ammonia, and other com-
pounds’ to absorb the basic nitroglycerin. |
By varying these materials, they can control |
both the sensitivity and the power of their |
dynamite and, at the same time, control |
fumes which will remain after detonation.
Such control is very important in preventing
accidents in underground mining.
Gelatin dynamites, containing nitrocotton
dissolved in the nitroglycerin, are highly |
water-resistant and can therefore be used |
for underwater blasting. In the old days,
many accidents were caused by attempting
to thaw frozen dynamite at open fires.
Today, antifreeze chemicals are mixed
into dynamite to eliminate this problem.
-
Language (Dublin Core)
-
eng
-
Date Issued (Dublin Core)
-
1944-05
-
pages (Bibliographic Ontology)
-
128-133
-
Rights (Dublin Core)
-
Public Domain (Google digitized)
-
Archived by (Dublin Core)
-
Lorenzo Chinellato
-
Federico Mazzini (supervisor)
 Popular Science Monthly, v. 144, n. 5, 1944
Popular Science Monthly, v. 144, n. 5, 1944
 Immagine 2022-05-05 142344.png
Immagine 2022-05-05 142344.png Immagine 2022-05-05 142401.png
Immagine 2022-05-05 142401.png Immagine 2022-05-05 142418.png
Immagine 2022-05-05 142418.png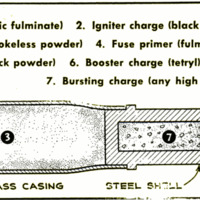 Immagine 2022-05-05 142448.png
Immagine 2022-05-05 142448.png Immagine 2022-05-05 142546.png
Immagine 2022-05-05 142546.png Immagine 2022-05-05 142602.png
Immagine 2022-05-05 142602.png Immagine 2022-05-05 142620.png
Immagine 2022-05-05 142620.png Immagine 2022-05-05 142633.png
Immagine 2022-05-05 142633.png Immagine 2022-05-05 142647.png
Immagine 2022-05-05 142647.png Immagine 2022-05-05 142702.png
Immagine 2022-05-05 142702.png