-
Titolo
-
The importance of nitrogen in war and in peace
-
Article Title and/or Image Caption
-
Title: Nitrogen in War and in Peace
-
Subtitle: It helped to make the world safe for democracy and it keeps us from starving
-
extracted text
-
NITROGEN, the most democratic
of all the elements, is the es-
sential factor without which
the war for democracy could not have
been won. In the last analysis, war is
an effort to discover which of two
sides can liberate the most nitrogen
where it will do’ the greatest damage.
For all explosives are nitrogen com-
pounds —their deadly effects the result
of the ineradicable tendency of this
liberty-loving gas to burst its honds
and hurl in every direction fragments
of whatever has served to restrain it.
From old-fashioned black powder to
the most modern and powerful trini-
trotoluol, or “T.N.T.,” nitrogen is the
basis of them all.
Even more essential, in peace, is the
possession of nitrogen in usable form.
Without its aid there could be no
plant growth, nor could animal life
upon this globe continue. Yet, while
nitrogen is literally as common as air,
since four fifths of the volume of the
earth's atmosphere is free nitrogen
(serving to dilute the essential oxygen
and make it breathable, so that you
would not be literally burned alive), the
problem of obtaining sufficient nitro-
gen is one that holds the serious, even
apprehensive attention of scientists,
economists, and the Government itself.
Before the world became densely
populated with people who live in
cities, and who therefore depend upon
the annual crops produced by others for
their food, instead of living on the
fruits, nuts, and game that were the
food of our ancestors, nobody worried
about nitrogen. People went where
food could be obtained; if they failed
to arrive soon enough they starved.
When the World Faced Starvation
Up to less than a hundred years ago,
the entire human race was constantly
menaced by the possibility of famine
and wholesale starvation, and nature's
methods of supplying nitrogen to
plants through the action of bacteria
in the soil long ago became too slow
to keep pace with the increasing de-
‘mand of the human race for food.
For a great many years the world has
been dependent for its supply of nitro-
gen for fertilizer upon enormous depos-
its of sodium nitrate, or Chile saltpeter,
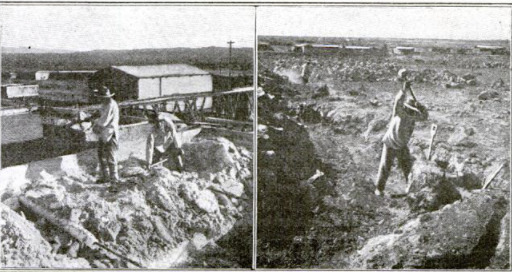
found in the high, arid desert regions
of Chile and Peru and nowhere else.
In late years there has been an ime
portant addition to this diminishing re-
source—the production of ammonium
sulphate as a by-product of the coking
of coal. But the total annual supply
from both of these sources, about
2,600,000 tons from each, is still in-
sufficient to meet the growing demand
for agricultural purposes alone, while
the war's demands created a situation
little short of critical.
Crookes’ Advice to Chemists
Twenty years ago Sir William
Crookes, then president of the British
Association, startled us by declaring
that the population of the world was
increasing so much faster than its
food supply that the race would soon
face starvation unless new means of
increasing the earth's fertility were
found. His words carried conviction,
and his suggestion that chemists turn
their attention to the development of
practical artificial means of extracting
the nitrogen of the air and “fixing”
it in usable compounds stimulated
experiment in that direction.
As a laboratory experiment, the
fixation of atmospheric nitrogen was
old. The main essential of all processes
then known, tremendously high tem-
peratures, running up even to 6,000°F.,
made the practical application of any
of them doubtful. Chemists, how-
ever, set to work. The development
of hydro-electric power at Niagara
and elsewhere, which made it possible
to produce high temperatures through
the electric arc, turned attention to
this’ means of accomplishing the
result sought. Charles S. Bradley, an
American engineer, almost at the time
Sir William Crookes was pointing out
the imminent need, began the first
large-scale experiment at Niagara Falls,
His process was not a commercial
success, but a little later a plant was
established at Notodden, Norway,
where, water-power costs only $3 a
year per horsepower. There nitrogen
Products were successfully made.
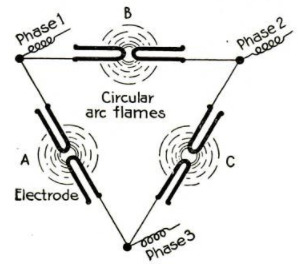
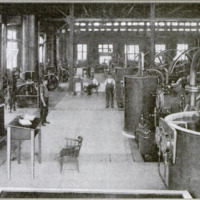
Reducing Nitrogen from the Air
The process used at Notodden and
later at several other plants in Norway,
known as the Birkeland-Eyde, is not
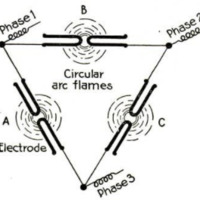
unlike that devised by Bradley. An
electric arc js produced by leading a
current of about 5,000 volts equatori-
ally between the poles of an electro-
magnet. This produces what is prac-
tically a disk of flame six and a half
feet in diameter and having a tempera-
ture of about 3,000° F. The disk
really consists of a series of successive
arcs which increase in size until they
burst. Air is passed through this arc.
The first, product of the reaction is
nitric oxide, which, on cooling with
the residual gases, produces nitrogen
peroxide. The cooled gases are then
led into towers, where they meet a
stream of water coming in the opposite
direction. Thus nitric acid is formed
in the towers, in diminishing degrees
of dilution. In the last tower the re-
‘maining gases are brought into contact
with milk of lime, which combines
with the gases to form calcium nitrate
and nitrite. The nitric acid obtained
in the other towers is combined with a
base to form a commercial compound.
These Norwegian plants were fi-
nanced by Germany, and their output
of fixed nitrogen was almost entirely
absorbed by that country. At the
beginning of the war the annual
production of the Norwegian plants
was equal to about 10,000 tons of
fixed nitrogen a year. At that time
Germany imported annually 880,000
tons of nitrate of soda, equal to 137,000
tons of nitrogen. At the beginning of
the war Germany had a stock of
1,000,000 tons of nitrate, equivalent to
156,000 tons of nitrogen, and a by-
product ammonia capacity of 550,000
tons of sulphate of ammonia, equiv-
alent to 113,000 tons of nitrogen.
It is known that the Norwegian
plants have not been commercially
successful. In Germany several other
processes for the fixation of atmos-
pheric nitrogen were developed, all
of which helped to supply the enor-
mous quantity of nitrogen products
required in manufacturing explosives.
One of these processes, developed
by two German scientists, Drs. Frank
and Caro, who began their experi-
ments in 1898, is known as the cyana-
mid process. ' It is based upon the
fact that caleium carbide, largely pro-
duced as a source of acetylene gas, may
be induced with comparative ease to
absorb nitrogen, thus forming a com-
bination of calcium, carbon, and nitro-
gen, known commercially as eyanamid.
‘This is the only process that has been
installed on the American continent,
a plant in Canada, at Niagara Falls,
having been in operation for several
years, with an annual capacity of
about 60,000 tons.
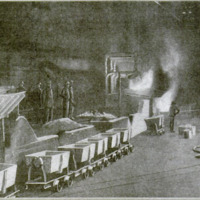
Production of Cyanamid
Thecarbideisplaced in the furnace and
the reaction is initiated by local resist-
‘ance heating to a temperature of 1500°-
2000° F., the conversion proceeding
to completion without further heating.
The nitrogen is obtained from liquid
air, manufactured by compressing air
to a density of 500 pounds to the square
inch and cooling by expansion. When
the liquid air begins to rise above its
normal temperature of —313° F., pure
nitrogen boils off. The compound of
calcium carbide and nitrogen, known
commercially as cyanamid, is itself
valuable as a fertilizer; by treatment
with superheated steam its nitrogen
may be released to enter into combina-
tion with the steam, forming am-
monia. In Germany about 600,000
tons of cyanamid is being produced
annually.
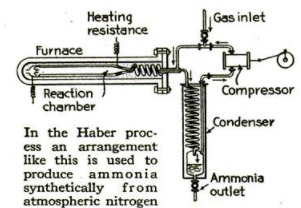
The other process of fixing atmos-
pheric nitrogen, on which Germany
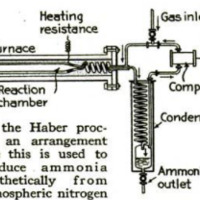
mainly relies, is the Haber process.
In this, nitrogen and hydrogen gases,
under a pressure of 1,500 pounds to the
square inch, are passed through a
chamber electrically heated to a tem-
perature of 1,170° F. As a result, the
nitrogen combines with the hydrogen
to form ammonia. Although this
process has long been in operation in
Germany, its technical details have
been carefully guarded, and it took
chemical and electrical experts em-
ployed by the United States Govern-
ment nearly a year to discover its
secret from patents obtainedgin this
country.
The Haber Process
The Haber process involves the
‘presence of what is known in chemistry
as a “catalyst.” It has
been found that certain
elements or compounds
— the list is constantly
being enlarged —have
the remarkable prop-
erty of causing other
substances to com-
bine chemically, often
in entirely new for-
mations, without
themselves under-
going any change or
entering into the new
combination. A fa-
miliar example is the
common device for
lighting gas without
matches, which con-
sists of a small bit of “spongy plat-
inum,” or asbestos, fibers coated with
platinum black. When this is held
over an open unlighted gas-burner,
the presence of the platinum causes
the hydrogen in the gas to combine
with the oxygen in the air with
such speed and violence that great
heat is generated by the reaction, the
spongy platinum becomes incandes-
cent, and in a second or two is so hot
that the gas ignites.
In the Haber process the catalyst
is spongy iron, although any one of
several other substances probably
would answer as well.
In the electric arc process the first
produet is nitric acid, which is directly
usable for explosives. In the cyana-
mid and Haber processes the ammonia
product is best adapted for use as
fertilizer, but is readily convertible
into nitric acid by passing a mixture
of ammonia and air through a red-hot
platinum screen acting as a catalyst.
The fact that nitrogen can be fixed
directly in the form of sodium cyanide
by the action of nitrogen gas on a
mixture of soda and coke has been
known for many years; but, while
English, German, and American scien-
tists have tried their hands on a com-
mercial adaptation of this reaction, it
is only recently that an American firm
has been able to prepare sodium
cyanide for the market by this process.
A Low-Temperature Process
Intensive study of the various meth-
ods of speeding up the reaction has led
to the adoption of special apparatus,
and, at a temperature around 1800° F,,
with the assistance of a specially de-
veloped catalyzer, an unusually pure
cyanide is formed. From the cyanide
it is easy to prepare ammonia quanti-
tatively.
One advantage of this sodium cya-
nide method of fixation, aside from the
low temperatures used,
is that when ammonia
is made from the cya-
nide, another product
of commercial value is
also obtained—sodium
formate. This latter
material can be used as
a starting-point for a
number of artificial fla-
voring oils, for a whole
line of useful solvents,
or for making the for-
mic and oxalic acids
that are so necessary
in our dyeing processes;
for instance, and which
were formerly imported,
chiefly from Germany.
-
Autore secondario
-
Frank Parker Stockbridge (Article author)
-
Lingua
-
eng
-
Data di rilascio
-
1919-01
-
pagine
-
39-41
-
Diritti
-
Public domain (Google digitized)
-
Archived by
-
Davide Donà
-
Marco Bortolami (editor)